Abstract
INTRODUCTION: Despite a significant increase in median overall survival (OS) over the past three decades, mantle cell lymphoma (MCL) remains incurable with no standard frontline therapy. The addition of R-DHAP to R-CHOP with autologous stem cell transplant (ASCT) in the European MCL Network - MCL younger trial showed significant improvement in median progression free survival (PFS), although some concerns remain regarding increased toxicity. On this basis, R-CHOP/R-DHAP was approved as first line therapy for MCL at CancerCare Manitoba (CCMB) in 2016. In contrast to the original study, R-DHAP is currently administered in an outpatient setting in Manitoba.
OBJECTIVE: To assess the real-world, non-trial PFS, OS, and toxicities of patients with MCL eligible for autologous stem cell transplant receiving R-CHOP/R-DHAP, administered on an outpatient basis, as induction therapy in Manitoba.
METHODS: All patients with MCL in Manitoba eligible for R-CHOP/R-DHAP induction between Jan 1, 2016 and Dec 31, 2021 were identified through the CCMB. Individual patient records were obtained and reviewed for baseline demographic data, laboratory data, treatment characteristics including response, and treatment toxicities. SPSS was then used for statistical analysis to calculate PFS and OS using the Kaplan-Meier method.
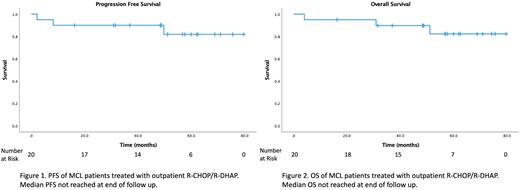
RESULTS: 25 charts were identified. 5 were excluded based on duplicate data for a total of 20 patients (85% male). The mean age at diagnosis was 54.4 (range 42-67). 75% had stage IV disease and 35% had constitutional symptoms. 15% of patients had ECOG ≥2 at diagnosis. 50% had elevated LDH at diagnosis, and the mean β-2-microglobumin was 3.5mg/L (range 1.9-16.1). 10% of patient's largest nodal dimension was >=7.5cm, 20% had disease with Ki67>=40%, and 15% had high risk disease by MIPI. The median number of chemotherapy cycles completed was 6 (range 2-6), with 50% requiring modification to therapy (5% progression, 20% infection, 20% ototoxicity, 20% nephrotoxicity, 5% corneal toxicity, 5% patient preference). 70% received ASCT, with 100% complete response by PET post-ASCT. 75% of all patients received maintenance rituximab, with a median of 8 cycles completed (range 2-8). At the end of follow up, there were 3 progression events, 3 deaths, and 1 death attributed to MCL. At a median follow up of 56.8 months, median PFS and OS were not reached.
CONCLUSION: Our real-world data show feasibility of administering R-CHOP/R-DHAP in the outpatient setting with comparable outcome and toxicities to original study where R-DHAP was administered as inpatient.
Disclosures
Kotb:Merck: Honoraria, Research Funding; Amgen: Honoraria; Akcea: Honoraria; BMS: Honoraria; Celgene: Honoraria; Takeda: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria, Research Funding; Karyopharm: Current equity holder in private company; Janssen: Honoraria.
Author notes
*Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal